Ultraviolet (UV) light is light with a range of wavelengths from 10 to 400 nm, shorter than visible light and longer than soft X-rays.
Generally speaking, "light" is a type of electromagnetic wave and refers to "visible light" with a wavelength of 380 to 780 nm that can be recognized by the human eye.
Infrared rays, which are emitted from heating devices around us and used as heat, and X-rays used in X-rays and CTs are also included in light.
Other electromagnetic waves include "radio waves" used in telecommunications, broadcasting, and other long-distance information communications, as well as gamma rays and other forms of radiation.
While infrared rays act thermally, ultraviolet rays are also called chemical rays because they have a significant chemical effect.
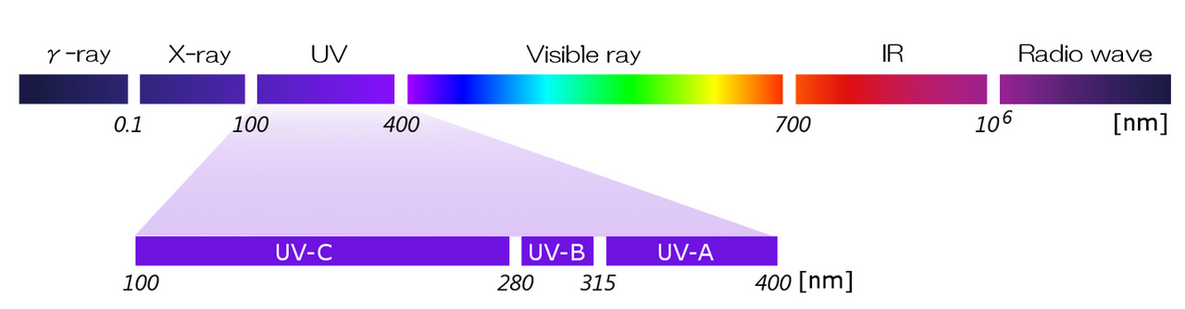
The following classifications by the Commission internationale de l'Eclairage (CIE) are known as familiar classifications of ultraviolet radiation.
| Wavelength (nm) | ||
| Long-Wavelength | UV-A | 315 ~ 400nm range |
| Medium Wavelength | UV-B | 280 ~ 315nm range |
| Short Wavelength | UV-C | 100 ~ 280nm range |
Ultraviolet rays are contained in sunlight, but shorter wavelength ultraviolet rays are absorbed by the atmospheric layer, and only ultraviolet rays above 280 nm (UV-A and UV-B) reach the earth's surface, where we live, and cause sunburn.The low-pressure mercury lamps integrated in our equipment clean and modify surfaces by irradiating ultraviolet rays in the shorter wavelength range, called UV-C. The relationship between wavelength and light energy is as follows: the shorter the wavelength, the greater the amount of energy, and the more effective the cleaning and surface modification.

Learn more about the relationship between wavelength and energy of UV light.
How Low-Pressure Mercury Lamps Work
Low-pressure mercury lamps are discharge lamps that use arc discharge in an environment with a mercury vapor pressure of 1 to 10 Pa. The wavelengths of ultraviolet rays emitted mainly are 184.9 nm and 253.7 nm, and they are made of quartz glass so that ultraviolet rays can penetrate easily.

Dangers of UV
The shorter the wavelength, the higher the energy of light. The energy of ultraviolet rays at 184.9 nm and 253.7 nm, the main wavelengths of low-pressure mercury lamps, is 647 kJ/mol and 472 kJ/mol, respectively.
Of the ultraviolet rays emitted by the sun, UV-C is absorbed by the atmospheric layer, and the ultraviolet rays that reach the ground are mainly those in the UV-A and some UV-B wavelength ranges.
For example, the energy at a wavelength of 400 nm (UV-A) is 299 kJ/mol, which is comparable to the high energy of low-pressure mercury lamps.
If ultraviolet rays emitted from low-pressure mercury lamps were to directly irradiate the human body, they would be highly dangerous, leading to serious burns and blindness.
Our UV irradiation equipment is designed to be safe to use, with a shielded structure around the UV lamps to prevent UV leakage, and a mechanism that automatically shuts off the lamps when the door is opened.