In UV cleaning, the short-wavelength light energy of ultraviolet rays emitted from low-pressure mercury lamps decomposes the bonds of organic contaminants on the substrate surface. At the same time, active oxygen (O) separated from ozone generated by the UV light chemically bonds with the organic contaminants and decomposes and reacts with volatile substances such as carbon dioxide and water to remove them. Generally, ozone has a cleaning effect on organic contaminants, but not on inorganic contaminants.
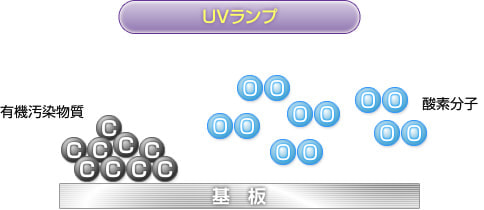
1) Before cleaning process
2) UV irradiation
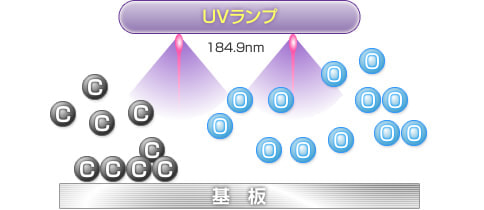
Ultraviolet rays at a wavelength of 184.9 nm emitted from UV lamps decompose oxygen molecules to produce oxygen atoms. The bonds of organic pollutants are also broken down.
3) Ozone generation

The decomposed oxygen atoms combine with O (oxygen molecules) in the atmosphere to generate O3 (ozone).
4) Generation of active oxygen
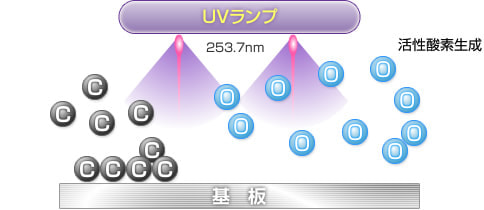
When the generated O3 (ozone) is irradiated with ultraviolet light at a wavelength of 253.7 nm, ozone is decomposed to generate O (active oxygen) in an excited state.
5) Removal of contaminants
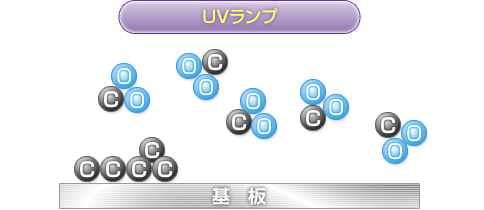
2) to 4) are repeated simultaneously, generating an abundance of active oxygen, which combines with the contaminant C (carbon) on the substrate to form volatile substances such as CO2, which are then eliminated.