Reactive oxygen formed by UV light strikes the substrate surface and cleaves the molecular chains of the surface layer, reacting with the cleaved molecules to generate new functional groups (OH, CHO, COOH, etc.). These functional groups are highly hydrophilic and compatible with paints, adhesives, coating agents, etc., resulting in a dramatic improvement and enhancement of adhesion.
1) Before surface modification treatment
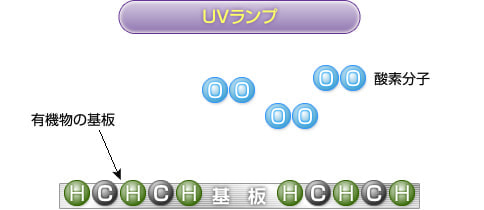
2) UV irradiation
 Ultraviolet rays at a wavelength of 184.9 nm emitted from a UV lamp decompose oxygen molecules to generate oxygen atoms. Most bonds on the substrate surface are also decomposed.
Ultraviolet rays at a wavelength of 184.9 nm emitted from a UV lamp decompose oxygen molecules to generate oxygen atoms. Most bonds on the substrate surface are also decomposed.
3) Ozone generation
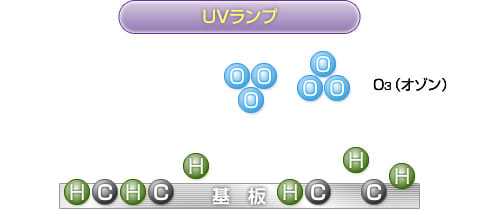 The decomposed oxygen atoms combine with O (oxygen molecules) in the atmosphere to generate O3 (ozone).
The decomposed oxygen atoms combine with O (oxygen molecules) in the atmosphere to generate O3 (ozone).
4) Generation of active oxygen
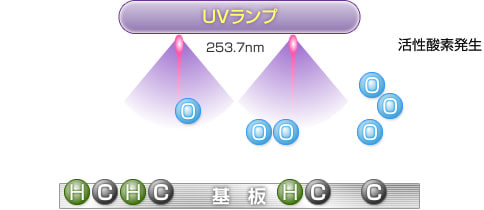 When the generated O3 (ozone) is irradiated with ultraviolet light at a wavelength of 253.7 nm, ozone is decomposed to generate O (active oxygen) in an excited state.
When the generated O3 (ozone) is irradiated with ultraviolet light at a wavelength of 253.7 nm, ozone is decomposed to generate O (active oxygen) in an excited state.
5) Continuous reaction
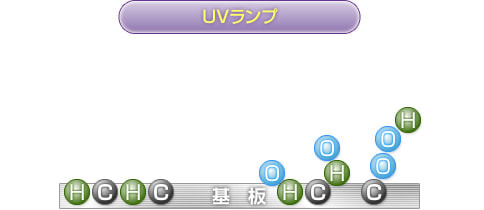 2) to 4) are continuously repeated, and the resin surface becomes very unstable after the bonds are broken down, and binds to the active oxygen present in the surroundings.
2) to 4) are continuously repeated, and the resin surface becomes very unstable after the bonds are broken down, and binds to the active oxygen present in the surroundings.
6) Surface modification
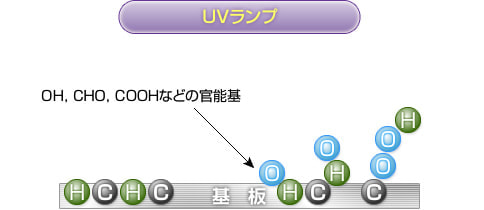 The resin surface to which the active oxygen binds develops into functional groups such as "OH, CHO, and COOH" and is modified.
These functional groups are highly compatible with paints, adhesives, and coatings, dramatically improving adhesion.
The resin surface to which the active oxygen binds develops into functional groups such as "OH, CHO, and COOH" and is modified.
These functional groups are highly compatible with paints, adhesives, and coatings, dramatically improving adhesion.